Boosting Healthcare with Integrated Research
Dedicated to advancing Healthcare in the Kingdom of Saudi Arabia and GCC
Empowering Clinical Research Excellence
Unleashing Innovations for Breakthrough Results
Complete management from study design and implementation to final reporting. We ensure rigorous planning and efficient execution to deliver high-quality, relevant results within the specified timelines, supporting your clinical research objectives.
Our non-interventional studies provide valuable data on the use of drugs and medical devices in real-world conditions, ensuring a deeper understanding of their effectiveness and safety. This approach supports informed decision-making and long-term product success.
We integrate patient needs through tailored programs, providing continuous support and actively engaging them in their care journey. These programs enhance patient adherence, improve health outcomes, and foster a patient-centric approach to treatment.
Submission and approval of authorization dossiers for all types of studies on health products, in full compliance with regulations in France, Europe, and globally. We ensure your products meet all regulatory requirements and monitor ongoing regulatory changes.
Comprehensive clinical data management and statistical analysis services, including data entry, verification, validation, data reconciliation, and advanced statistical analyses. We ensure accurate data handling and deliver insights that support informed decision-making.
Our market access services help you navigate the complexities of local and international regulations, ensuring the efficient and compliant entry of your products into the market. We streamline the process to support timely approvals and long-term market success.
We offer advisory and writing services for scientific publications, including literature reviews, abstract writing, poster creation, article drafting, and presentation materials. Our expertise ensures clear, accurate, and impactful communication of your research findings.
Specialized programs in clinical research, pharmacovigilance, medical writing, and regulations. Each course is tailored to meet specific needs, ensuring a deep understanding of current practices and strict compliance with applicable standards and guidelines in the healthcare industry.
Experience, Expertise, and Excellence: Our Track Record Speaks Volumes
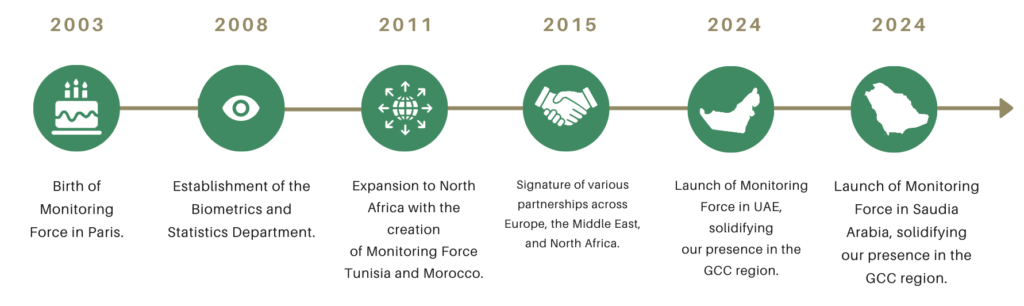
At Monitoring Force, we don't just conduct clinical research, we work with sponsors to propose the project that meets their needs. Our expertise goes beyond conducting studies and focuses on proposing the right study. Our key figures reflect our global experience and diverse expertise. The story of our development during these last 20 years explains our unwavering commitment to meeting the unique needs of the GCC region. We're more than a CRO - we're a trusted partner, committed to delivering the project that meets your objectives, and results that exceed expectations. With us, your project is in the hands of leading experts who understand your challenges and share your goals.
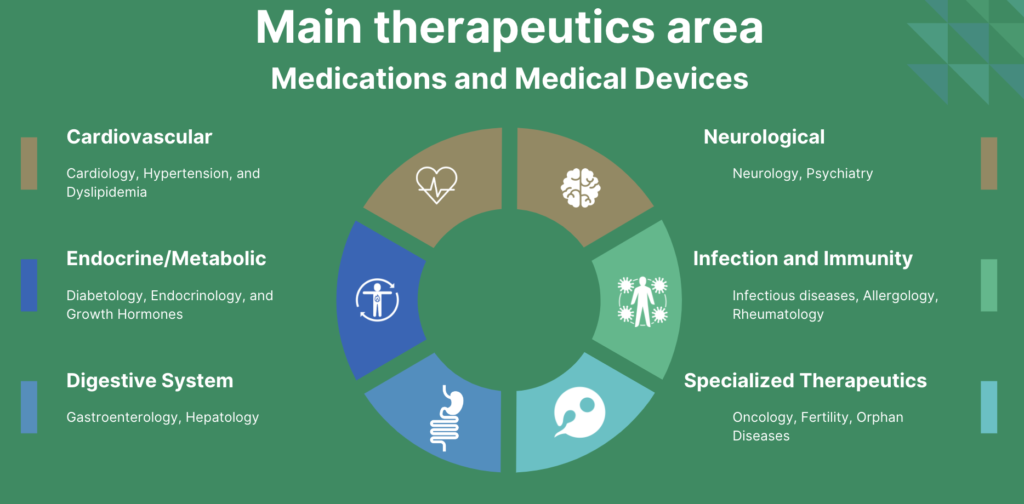
Therapeutic areas
Our Customers
More than 5O pharmaceutical companies have trusted us








Team Of Experts

Dr. Amin Kadi
President, Monitoring Force
With over 30 years of combined experience in cardiology and CRO activities, Dr. Amin Kadi epitomizes the blend of medical expertise and industry leadership, driving innovations and excellence at the forefront of healthcare research.

Samy Aloulou
General Manager, Monitoring Force Gulf
brings 15 years of seasoned experience in business and operational development. His strategic vision and leadership have been pivotal in the growth and success of Monitoring Force Gulf, setting new standards in the industry.

Baptiste
Project Director
With a decade of project management in the CRO sphere, Baptiste is known for his meticulous approach and results-driven leadership. His expertise in steering complex projects has been instrumental in delivering exceptional outcomes for our clients.

Why choose Monitoring Force Gulf ?
In a rapidly evolving healthcare landscape, choosing the right partner for your clinical research needs is more important than ever. At Monitoring Force Gulf, we understand the complexities of this field and have the expertise, experience, and dedication to help you navigate it successfully. Here are just a few reasons why leading organizations choose us as their trusted partner in clinical research.
Global Experience, Local Expertise
With over 280 projects conducted around the world, we bring a wealth of global experience to every project. But we also understand the unique needs and challenges in each region where we have to conduct our project as in the GCC region. We combine these two strengths to deliver solutions that are both world-class and locally relevant.
Comprehensive Service Offering
From design to project management, analysis and publication, we offer a complete range of services. This means we can handle every aspect of your project, saving you time, avoiding multiple service providers and ensuring consistency and quality at every stage.
Proven Track Record
Our key figures speak for themselves. With 75 publications and 62 satisfied customers, we have a proven track record of delivering results. When you work with us, you can be confident that your project is in safe and capable hands.
Trusted Partner
We see ourselves as more than just a CRO – we are a trusted partner to our clients. We work closely with you to understand your vision and goals, and we are committed to exceeding your expectations. With Monitoring Force, you’re not just getting a service provider – you’re getting a partner who is invested in your success.
What Our Clients Say About Monitoring Force?
Nothing speaks more to our commitment, expertise, and impact than the words of our clients themselves. These testimonials reflect the trust we've earned and the partnerships we've built. Discover why organizations across the globe choose Monitoring Force as their trusted partner in clinical research.
"Working with Monitoring Force has been a game-changer for our operations. Their expert advice and practical solutions have helped us navigate complex scientific challenges with ease."

CEO, ZIWIG
"Working with Monitoring Force Gulf has been a game-changer for our clinical research initiatives. Their innovative approach and unwavering commitment to quality have significantly enhanced our projects. They are not just a service provider, but a true partner in healthcare innovation."

Head of Global Medical NMD -Biogen
"The expertise and professionalism of the Monitoring Force Gulf team have been instrumental in the success of our recent clinical trials. Their attention to detail and proactive communication made a complex process feel seamless. They are a beacon of excellence in healthcare research."

Professor, Østfold Hospital and Institute of Clinical Medicine
"Collaborating with Monitoring Force Gulf was an exceptional experience. Their deep understanding of the healthcare landscape in the GCC region, coupled with cutting-edge technology, brought invaluable insights to our project. They truly stand out in their field."

CEO, NEXTKIDNEY
Expertise & Personalized Support
At Monitoring Force Group, we offer tailored support for each clinical research project. Our dedicated team ensures rigorous planning and effective execution to guarantee reliable and relevant results.
- Personalized Approach
- Scientific Excellence
- Innovative Technologies
- Continuous Support and Adaptability
- Regulatory Compliance
- Training and Education



Study Case :
ZIWIG
The client’s objective was to launch a pivotal study on an RNA signature-based diagnostic tool for endometriosis. It needed to validate this signature on a large cohort in order to obtain market authorization for a high-impact scientific innovation in a relatively short timeframe. Indeed, only rapid results would enable him to raise the funds needed to continue his development.

The Challenge
The customer expected Monitoring Force to propose a study design with an objective and a primary endpoint that would enable it to meet the authorities' expectations. It expected to obtain regulatory approval, select centers, set up and monitor the study, collect data and provide a clinical report. All within a very tight timeframe. It was understood that only irreproachable quality could make the study a success.
Lessons Learned
During this project, we had to face many challenges with 1/ Very short deadlines that we were able to meet by setting up a communication and decision-making circuit with the sponsor very early on, by establishing the role and responsibilities of each member of the project team and above all by setting up a rescue plan for each gap between the theoretical and actual inclusion curve. 2/ Logistical difficulties in the transmission of samples on time and in compliance with quality requirements. This challenge was met by training the teams on site and by choosing a qualified transporter.
Approach Adopted
Monitoring Force designed a project that took into account the regulatory requirements and expectations of the authorities. The drafting of the protocol was the subject of lengthy reflection combining methodological excellence and perfect mastery of the pathology by renowned experts interviewed by Monitoring Force. The process of selecting the investigators involved an in-depth analysis of the medical and technical capabilities of the centers to be selected, as well as Pre Study Visits. As with all of our projects, this involved a combination of skills to ensure success, including a doctor specialized in clinical research, a regulatory affairs manager, a medical editor, a project manager, a data manager, a statistician and a CRA.
Results Achieved
The results of the study were tremendously positive, and we were able to provide the customer with over 70 statistical tables presenting all the results. All these tables were interpreted and integrated into a clinical report forwarded by the customer to the authorities.
Our Success Indicators
Explore the key indicators that reflect our continuous growth, expertise, and global impact.
